SHANGHAI, Feb. 23, 2025 /PRNewswire/ — On February 15, 2025, Sanyou Bio conducted a full review of its Intelligent Super-trillion Molecule Discovery Platform. As the core technology of the platform, the intelligent super-trillion molecule library boasts a library capacity of up to 10 trillion, enabling the screening and identification of hundreds to thousands of lead molecules for a single target. By integrating high-through eukaryotic expression verification and multidimensional druggability analysis, the platform significantly improves the efficiency of innovative biological drug R&D and plays a key role in novel macromolecule drug discovery.
Sanyou Bio’s Intelligent Super-trillion Molecule Discovery Platform consists of ten sub-platforms, encompassing a variety of molecular types. These include super-trillion fully human antibody discovery platform, super-trillion common light chain antibody discovery platform, super-trillion 2C type single-domain antibody discovery platform, super-trillion 4C type single-domain antibody discovery platform, super-trillion cyclic peptide discovery platform, super-trillion novel targeting protein discovery platform, magnetic-array mouse immune antibody discovery platform, magnetic-array alpaca immune antibody discovery platform, magnetic-array rabbit immune antibody discovery platform, and magnetic-array canine immune antibody discovery platform.
Details of the ten sub-platforms are summarized in Table 1. Each sub-platform has unique characteristics and can be widely applied in therapeutic, diagnostic, detection and scientific research fields. So far, the Intelligent Super-trillion Molecule Discovery Platform has completed over 650 molecule discovery projects, advancing more than 100 PCC projects, with several projects have entered the clinical stage. This further demonstrates the capability of Sanyou Bio’s platform to complete the development of mainstream drug molecules such as monoclonal antibody drugs, bispecific antibody drugs, multi-specific antibody drugs, ADC drugs, AOC drugs, RDC drugs, PDC drugs, and CAR-T immune cell therapy.
Table 1. Applications and Advantages of the Intelligent Super-trillion Molecule Discovery Platform
| No | Library | Library | CDR3 | Characteristics |
| 1 | Super-trillion fully | 2.16 x 1012 | 5~28 | Direct acquisition of fully human antibody sequences with |
| 2 | Super-trillion | 1.12 x 1012 | 4~26 | Common light chain with excellent druggability and high |
| 3 | Super-trillion 2C | 2.13 x 1012 | 12~19 | Humanization level up to 98%, enabling the discovery of |
| 4 | Super-trillion 4C | 1.94 x 1012 | 14~21 | Humanization level up to 98%, with excellent molecule |
| 5 | Super-trillion cyclic | 3.05 x 1012 | 4~17 | High diversity; optimized fusion with tag proteins for |
| 6 | Magnetic-array | 7.10 x 1011 | 4~15 | Fast screening speed, the process is nearly three months |
| 7 | Magnetic-array | 2.83 x 1011 | 3~27 | Capable of obtaining a large number of antibody |
| 8 | Magnetic-array | 4.88 x 1010 | 6~20 | Abundant types of immune raw materials and diverse |
| 9 | Magnetic-array | 4.21 x 109 | 5~20 | Direct acquisition of canine-derived monoclonal |
| 10 | Super-trillion novel | 6.00 x 1010 | 4~8 | Stable protein backbone structure with low |
Part 1 Review of the Intelligent Super-trillion Molecule Discovery Platform
1. Super-trillion fully human antibody discovery platform
Library background and history
Phage display technology is a crucial way to develop fully human antibodies, demonstrating advantages over hybridoma antibody technology in many aspects. Currently, human phage display antibody libraries on the market face challenges such as limited donor sources, low library capacity, low diversity, low affinity, and low druggability. To address these issues and obtain more candidate antibodies with better quality, Sanyou completed the conceptual design of the sub-trillion fully human antibody library in 2017, focusing on antibody backbone sequences with higher druggability and avoiding the presence of pseudogenes at the same time. By 2018, the sub-trillion fully human antibody library was constructed and validated. To further expand the library capacity, Sanyou completed the conceptual design of the super-trillion fully human antibody library and the super-trillion fully human semi-synthetic antibody library in 2019 and 2020 respectively. By 2021, both antibody libraries were constructed and validated.
Library composition and characteristics
Sanyou’s super-trillion fully human antibody discovery platform consists of two major libraries: super-trillion fully human antibody library and super-trillion fully human semi-synthetic antibody library, with a total library capacity of 2.16 trillion. The super-trillion fully human antibody library is a recombinant antibody library after gene cloning of human B cells. It is derived from nature with excellent druggability and the median number of lead molecules obtained is 300+. The super-trillion fully human semi-synthetic antibody library is derived from human B cells after gene rearrangement with engineering design. It offers high diversity with a median number of lead molecules obtained also at 300+.
Library cases and applications
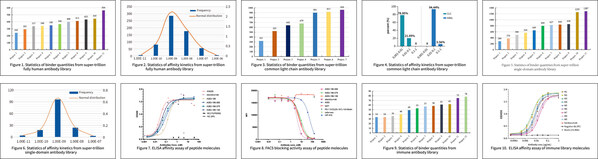
So far, Sanyou has completed over 200 single-target screenings using fully human antibody library. The lead molecules obtained from super-trillion fully human antibody library can reach hundreds. As shown in Figure 1, through screening and validation across 11 different targets, a total of 5,069 fully human antibody clones with unique sequences were obtained, and the median number of clones was 345 per target. The affinity of molecules screened from Sanyou’s super-trillion fully human antibody library often ranges from sub-nM to nM levels. Figure 2 shows the affinity analysis of expressed supernatant samples. The results reveal that the majority of antibody clones exhibit affinities in the 10⁻⁸ to 10⁻¹⁰ range.
2. Super-trillion common light chain antibody discovery platform
Library background and history
Bispecific antibodies with a common light chain configuration can effectively solve the issue of light-heavy chain mismatch, and are highly consistent with natural IgG-type antibodies in structure. Compared with various non-human bispecific antibody configurations, bispecific antibodies with a common light chain configuration offers better druggability and safety profiles in human body. Sanyou completed the preliminary conceptual design of the common light chain antibody library, and conducted preliminary research on common light chain antibodies in 2017. Then completed the construction of the billion-level common light chain antibody library in 2018, and the validation of the billion-level common light chain antibody library in 2019. In 2020, the concept design of the super-trillion common light chain antibody library was completed, and 2 exceptional light chains were selected from over 1800 light chains through rigorous screening. The construction and validation of the super-trillion common light chain antibody library was completed in 2022.
Library composition and characteristics
Sanyou’s super-trillion common light chain antibody discovery platform is composed of 2 light chains and 6 sub-libraries with a total library capacity of 1.12 trillion. It is a recombinant antibody library by selecting 2 exceptional light chains paired with a diverse set of heavy chains. The library is derived from fully human natural antibodies, with carefully selected light chains for optimal performance and diverse heavy chains. The median number of lead molecules obtained is 300+. Common light chain antibodies targeting different epitopes of the same target or different targets can be screened in one step; the obtained common light chain antibodies are fully human antibodies and can be directly used for downstream bispecific antibody development.
Library cases and applications
So far, Sanyou has completed over 30 screenings using common light chain antibody library. For different targets, an average of hundreds of lead antibody molecules with unique sequences and good affinity can be obtained. As shown in Figure 3, through screening and validation across 7 different targets, a total of 4,942 common light chain antibody clones with unique sequences were obtained, and the average number of unique sequence clones was 706 per target. The binding activity of candidate antibodies obtained from the human library (hRAL) and the common light library (CLC) was detected by ELISA. Figure 4 reveals that the proportion of hRAL candidate antibodies with EC50 between 0.01 and 0.1 was 94.44%, while the proportion of CLC candidate antibodies with EC50 between 0.001 and 0.01 was 78.95%, indicating CLC antibodies have higher affinity.
3. Super-trillion single-domain antibody discovery platform
Library background and history
Humanization of single-domain antibodies can significantly reduce the immunogenicity of camel-derived antibodies and accelerate the production and commercialization of antibody-based drugs. To overcome the defects of traditional single-domain antibody preparation technology, such as long preparation cycles, heavy screening workloads, and small number of lead candidates, Sanyou’s super-trillion single-domain antibody discovery platform has undergone four generations of library upgrades: completion of the construction and validation of the 10-billion single-domain naïve library in 2018; completion of the construction and validation of the sub-trillion single-domain antibody library in 2019; completion of the humanized single-domain conceptual design using a humanized backbone for library construction, and at the same time design of sub-libraries with only 1 pair of cysteines and 2 pairs of cysteines; completion of the construction and validation of the super-trillion humanized single-domain antibody library in 2021.
Library composition and characteristics
Sanyou’s super-trillion single-domain antibody library is constructed using internationally advanced primer synthesis technology and a humanized backbone with excellent druggability, ensuring superior therapeutic potential. This platform has independent intellectual property rights and is supported by comprehensive physicochemical and biomedical analyses for efficient screening. The platform comprises two major sub single-domain synthetic libraries 2C-type and 4C-type. There are further divided into 16 sub-libraries with a total library capacity of 4.07 trillion. The humanization degree of the antibody backbone reaches 98% and the median number of lead molecules obtained is over 600.
Library cases and applications
So far, Sanyou has completed over 60 screenings using single-domain antibody library. As shown in Figure 5, through screening and validation across 14 different targets, a total of 9,423 humanized single-domain antibody clones with unique sequences were obtained, and the median number of clones was 606 per target. The affinity of nanobody candidate molecules screened from Sanyou’s super-trillion humanized single-domain antibody library has excellent affinity and often reaches pM levels. Figure 6 shows the affinity analysis of full-length constructed molecules. The results reveal that the majority of antibody clones exhibit very high affinities.
4. Super-trillion cyclic peptide discovery platform
Library background and history
According to statistics, the global peptide drug market reached $62.8 billion in 2020 and is expected to grow to $96 billion by 2025, highlighting the significant growth potential in this sector. Peptide-based drugs offer the advantages of high specificity, low immunogenicity, less toxic side effects, and the applicability to intracellular targets. The establishment of peptide libraries is crucial for peptide drug R&D, but constructing such libraries requires substantial time and financial investment for most drug R&D companies. To address this challenge, Sanyou completed the construction and validation of the sub-trillion cyclic peptide library in 2022 and further advanced to the super-trillion cyclic peptide library in 2023, significantly enhancing the efficiency and success rate of peptide drug discovery.
Library composition and characteristics
Sanyou’s super-trillion cyclic peptide library follows natural design principles, supplemented by AI-driven design, fused with tag proteins, combined with phage display technology for high-throughput screening, and matched with comprehensive physicochemical and biochemical analyses for further validation. The library has a total of 19 sub-libraries, with peptide lengths ranging from 4 to 17 amino acids and a total library capacity of 3.05 trillion. The cyclic peptide platform integrates optimized tag protein with natural cyclic peptide and is suitable for PDC, oral, and intracellular target drug development, with a median number of lead molecules obtained of 60+.
Library cases and applications
So far, Sanyou has completed over 20 screenings and validation using cyclic peptide library. As illustrated in Figure 7, the peptide molecule A083, obtained from the super-trillion cyclic peptide library, underwent affinity maturation and was evaluated for ELISA binding activity using huPD-L1 antigen protein. The EC50 of antigen-binding was approximately 0.04 nM, comparable to the affinity of the reference antibody. Additionally, A083 demonstrated a 10-fold improvement in blocking activity, compared with the reference antibody. Figure 8 reveals that the EC50 for blocking the interaction between huPD1 and huPD-L1-CHO-K cells was 5.7 nM,
5. Magnetic-array immune antibody discovery platform
Library background and history
Sanyou Bio established its proprietary phage display vector platform in 2015, and applied the "multi-pathway antigen and antibody library" technology to the development of innovative antibody drugs. In 2016, we integrated hybridoma technology and phage display technology to promote the commercialization of the mouse immune antibody library. In 2017, the mouse immune library underwent a significant upgrade by incorporating array-based primers to further improve library diversity. The alpaca immune antibody library was launched in 2018, and afterwards, the rabbit immune antibody library and the canine immune antibody library were established in 2023 and 2024 respectively.
Library composition and characteristics
Sanyou’s magnetic-array immune antibody library includes: mouse immune antibody library, alpaca immune antibody library, rabbit immune antibody library and canine immune antibody library, with a total library capacity of 1.05 trillion. The specific parameters of each immune library are shown in Table 2. Antibodies can be produced for different target types (including small molecules). The number of nanoantibody lead molecules obtained through the screening of the Sanyou magnetic array immune antibody library is large, usually reaching dozens of molecules, with high molecular affinity. The library has broad target coverage, capable of screening antibodies against diverse targets, including small molecules and high yield of lead molecules, typically dozens per target with excellent affinity.
Table 2. Technical specification of the magnetic-array immune antibody library
| No | Library | Library | Targets | Characteristics |
| 1 | Magnetic-array | 7.10 x 1011 | 263 | 1. Direct acquisition of antibody sequence to 2. Multi-point and diverse immunization, easy to 3. Capable of delivery of dozens to hundreds of |
| 2 | Magnetic-array | 2.83 x 1011 | 180 | 1. Defined antibody sequence to reduce 2. Capable of delivery of dozens to hundreds of 3. Coverage on high-difficulty and hidden- |
| 3 | Magnetic-array | 4.88 x 1010 | 36 | 1. Single subtype, easy to humanize 2. Diverse immunization strategy, easy to obtain 3. Suitable for diagnostic antibody development |
| 4 | Magnetic-array | 4.21 x 109 | 10 | 1. Fill the gap in the pet monoclonal antibody 2. Simplify the technical difficulty of 3. Canine monoclonal antibodies have a longer |
Library cases and applications
So far, Sanyou has completed over 480 screenings using immune antibody library. As illustrated in Figure 9, through screening and validation across 12 different targets, a total of 637 antibody clones with unique sequences were obtained, and the median number of clones was 55 per target. The affinity of antibody molecules screened from immune antibody library construction and screening service usually reaches pM levels. Figure 10 shows the affinity of the molecules screened from immune antibody library is comparable to or significantly better than that of the reference antibody.
Part 2 Development of the Intelligent Super-trillion Molecule Discovery Platform
1. Construction plan of the 100-trillion molecule library platform
Sanyou Bio remains committed to its mission of "making innovative biologics easier worldwide". Building upon the foundation of the Intelligent Super-trillion Molecule Discovery Platform, we are now advancing the development of the 100-trillion Molecule Library Platform, driven by R&D and innovation to promote the development of the specialized services. Based on the current molecule library, the 100-trillion molecule library in future will achieve: 1) the library types will be expanded on top of the existing 10 sub-libraries to further diversify by incorporating nucleic acid libraries, small peptide libraries, and small molecule libraries. By 2027, the total library capacity is projected to reach 100 trillion; 2) the design of the library will be further optimized through AI assistance, the molecule diversity and performance will be further improved based on massive data analysis, and the customized construction of novel molecule library will be carried out; 3) through AI-driven drug screening, the screening efficiency will be accelerated, the R&D cycle will be shortened, and the success rate of new drug discovery will be enhanced.
2. Construction plan of the intelligent drug screening platform
The intelligent drug development platform aims to revolutionize antibody drug discovery through virtual screening and design optimization technology powered by generative AI models. This innovative approach will significantly improve drug development efficiency, and help solve the current issues of long antibody drug development cycle, high cost and low success rate. The intelligent drug development platform will greatly shorten the drug development cycle and enhance the success rate.
The intelligent drug screening platform plans to further develop AI-driven drug screening and other applications based on multiple applications such as the oneClick+ bispecific antibody design platform that have been launched. Main applications include: 1) De novo molecule generation, by utilizing AI algorithms to design and generate of molecular structures with specific properties and functionalities. Break through the traditional discovery model, enabling the design and development of first-in-class drugs, and expand the scope and possibilities of drug development; 2) Coding sequence optimization, through AI-powered sequence optimization to enhance the druggability and stability of the molecules; 3) Innovative molecule library design, based on AI-driven big data analysis to design and construct next-generation molecule libraries with improved efficiency and success rate of drug screening and discover more molecules with potential application value. This will further solve the drug development issues of high-difficulty targets such as GPCRs, ion channels, and intracellular proteins, and develop new drug delivery methods such as oral administration. The rapid development of these technologies enables us to discover drug targets with higher efficiency and shorter time, create biologics that meet clinical needs, and respond to diverse scientific and medical challenges.
3. Prospects of the development of the intelligent super-trillion molecule library
Traditional drug discovery often faces challenges such as long development cycles and high costs. In contrast, the intelligent super-trillion molecule library platform, powered by AI-driven technologies, accelerates the discovery and development process, reduces costs, and enhances efficiency.
Looking into the future, Sanyou Bio’s intelligent super-trillion platform will develop in a more intelligent direction. In terms of development direction, it explores towards 5 major directions, including indications, molecular formats, application scenarios, mini-programs, and important targets. In terms of molecular types, it covers 6 types of molecules, including fully human monoclonal antibodies, common light chain antibodies, humanized single-domain antibodies, cyclic peptide molecules, ADC molecules, and mRNA molecules, proving diversified molecular tools for the treatment of different diseases. In terms of application scenarios, it is adapted to 8 key scenarios, including ELISA, WB, IHC, IF, IP-ID, FC, SPA, and SPB. In terms of technology integration, 7 AI-driven programs are integrated, including de novo generation, epitope prediction, immunogenicity prediction, druggability prediction, specificity prediction, affinity predication, and one-click molecule design, to improve the intelligence level of R&D. In terms of disease research, it focuses on 9 major human diseases, including tumors, autoimmune, infection, metabolism, ophthalmology, neurology, cardiovascular, anti-aging, and various rare diseases. In terms of target exploration, it concentrates on in-depth research over 100K important targets by integrating 10 major technology platforms to lay a solid foundation for the development of more innovative drugs.
After 10 years of relentless effort and technological innovation, Sanyou Bio’s intelligent super-trillion molecule library platform has established a world-leading antibody drug discovery system. With the design of super-trillion molecule library and ten categories of molecule libraries, the platform has successfully solved the key difficulties in the discovery of innovative macromolecule drugs and has broad application prospects, especially in the fields of tumor treatment, infectious disease prevention and treatment, autoimmune diseases and rare diseases. The ability of high-efficiency screening, precise optimization and rapid evolution provides strong support for the development of global antibody drugs. In the next five years, the current library will continue to be upgraded to the 100-trillion molecule library. Combined with AI power and high-throughput screening technology, it will be able to predict the properties of drugs more accurately, thus significantly improving the efficiency and success rate of drug development. This will greatly expand our space for exploring potential drug molecules and accelerate the process of new drug discovery. Sanyou Bio will continue to promote in-depth collaboration with professional teams globally to jointly accelerate the process of drug R&D in high-difficulty disease areas and bring more breakthrough treatment options to patients worldwide.
About Sanyou Bio
Sanyou Biopharmaceuticals Co., Ltd. is a world-leading high-tech biotechnology enterprise focusing on R&D and services of innovative biologic drugs. Sanyou has built the 4C business patterns that integrate "differentiated CRO, integrated CDO, innovative CPO and characteristic CRS", to accomplish the mission "to make the R&D easy for innovative biologics".
Sanyou has established an integrated innovative biologic drug R&D laboratory with advanced facilities, and has a professional team with the majority holding a Ph.D. or master degree. Sanyou has built three industry-leading innovative technology platforms featured by "super-trillion, integration, and intelligence" , which are comprised of more than 50 sub-platforms with the core innovative super-trillion phage display platform, and supported by platforms of material preparation, biologics discovery, molecule optimization, in vitro and in vivo efficacy, production cell line construction, upstream and downstream process development, preclinical R&D, industrialization development, etc.
Sanyou’s business network has expanded to all parts of the world, including Asia, US and Europe, and established branches in Boston, Philadelphia, San Diego and London. Sanyou has established friendly business relationships with more than 1000 pharmaceutical companies, drug R&D institutions and diagnostics companies worldwide. Sanyou received National-level certification as a high-tech enterprise and a Specialized and Sophisticated enterprise, and passed the ISO9001 quality assurance certification and GB/T intellectual property management system certification.